Overview
The normal ovary by nature is a partially cystic structure. Most ovarian cysts develop as consequence of disordered ovulation in which the follicle fails to release the oocyte. The follicular cells continue to secrete fluid and expand the follicle, which over time can become cystic.
The ovaries are the female pelvic reproductive organs that house the ova and are also responsible for the production of sex hormones. They are paired organs located on either side of the uterus within the broad ligament below the uterine (fallopian) tubes. The ovary is within the ovarian fossa, a space that is bound by the external iliac vessels, obliterated umbilical artery, and the ureter. The ovaries are responsible for housing and releasing ova, or eggs, necessary for reproduction. For more information about the relevant anatomy, see Ovary Anatomy.
Indeed, ovarian cysts were the fourth most common gynecologic cause of hospital admissions according to a late 1980s study by Grimes and Hughes. [1] Most cysts spontaneously resolve while some will persist. The persistent ovarian cysts are most likely to be surgically managed. The standard surgical approach to presumptively benign ovarian cysts is the laparoscopic ovarian cystectomy. Indeed, it is one of the most common procedures performed by the practicing obstetrician gynecologist.
In this article, the pathophysiology of ovarian cysts is briefly reviewed to provide a foundation for understanding the ovary and benign cyst formation. The remainder of the article concentrates on the patient evaluation and surgical approaches to cyst removal.
Pathophysiology of ovarian cyst formation
Obstetrician-gynecologists and surgeons most commonly encounter 3 types of benign ovarian cysts. They include functional (follicular and corpus luteum) cysts, mature cystic teratomas, and endometriomas. Functional cysts form in reproductive-aged females during folliculogenesis and are either follicular or corpus luteal in origin.
The cysts occur during the process of normal female reproductive physiology, hence their functional designation. The pathogenesis of follicular cyst formation is complex and is associated with the release of anterior pituitary hormones. In these cases, the traditional feedback mechanisms are not synchronized and the luteinizing hormone surge is muted. [2]
Consequently, the oocyte is not released by the follicle, which in turn fails to involute and continues to grow, sometimes achieving cystic proportions. Corpus luteum cysts develop after ovulation through an unknown mechanism. They can become quite large and torsed and, thus, are more likely to be associated with pain and in some cases delayed menses. Some cysts autonomously function such as those associated with the McCune-Albright syndrome and can achieve large sizes.
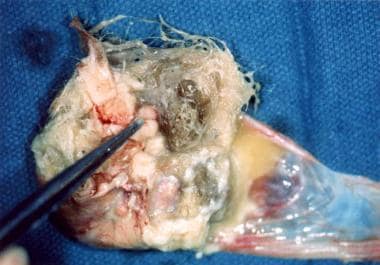
Mature cystic teratomas (MCTs) or dermoids are actually benign germ cell tumors that are partially cystic. They can occur over a broad range of ages, yet more than 70% occur during the reproductive years. [3] They are thought to develop from a single primordial germ cell that has completed meiosis I and is meiosis II-suppressed. [4] This theory is supported by the anatomic distribution of teratomas throughout the migration pathway of primordial germ cells from the yolk sac to the gonadal ridges. [5] MCTs are composed of all 3 germ layers: ectoderm, mesoderm, and endoderm. They are usually unilateral, measure 2-4 cm in diameter, and are filled with thick sebaceous material, hair, calcifications and sometimes teeth (see images below). [6] Some are even hormonally active. [7]
Unlike simple cysts, MCTs do not resolve spontaneously. Most require surgical intervention. They are more likely than other benign cysts to be associated with ovarian torsion. Although dermoid cysts are benign, complications from their rupture include chemical peritonitis, bowel adhesions and obstruction, and abscesses. [8]
Endometriomas are hormonally active ovarian cysts with the hormone changes corresponding to menstrual cycle phases. The origin of endometriomas has been controversial. Nezhat and coworkers have suggested that 2 types of endometriomas exist: primary and secondary. [9] According to the authors, primary endometriomas originate as invaginated surface endometrial glands. They develop slowly over time and rarely achieve sizes greater than 5-6 cm. They are quite difficult to remove from their fibrotic capsule at the time of cystectomies. Upon microscopic examination, both endometrial glands and stroma are identified. Secondary endometriomas originate in functional cysts, with some having their origins in a corpus luteum. These endometriomas are the classic chocolate cysts and contain dark blood (see image below). Secondary endometriomas can achieve quite large sizes and can be removed easily. Microscopic examination of a well-sampled specimen often reveals a corpus luteum, endometrial glands, and stroma. [9]
Epidemiology
Ovarian cysts are quite common and involve all age groups, occurring in both symptomatic and nonsymptomatic females. [10] Six percent of 5000 healthy women in a study reported by Campbell et al had detectable adnexal masses on transabdominal ultrasound. Of these, 90% were cystic with most diagnosed as simple cysts. [11] About 8% of premenopausal women develop large cysts that need treatment. According to National Institutes of Health estimates, 5-10% of women have surgery to remove an ovarian cyst. Of these cysts, approximately 13-21% are cancerous.
A 2-year interim analysis from the International Ovarian Tumor Analysis Phase 5 (IOTA5) study showed that 80% of ovarian cysts considered benign on ultrasonography either disappeared or required no intervention. Only 12 of the 1919 women in the study received a diagnosis of ovarian cancer; thus, the 2-year cumulative risk of cancer was 0.4%. [12]
Ovarian cysts are less common after menopause. Postmenopausal women with ovarian cysts are at higher risk for ovarian cancer. [13] A systematic review and meta-analysis by Liu et al found that the malignancy rate (including borderline tumors) for simple ovarian cysts in postmenopausal women was approximately 1 in 10,000. [14]
Indications
Absolute indications for ovarian cystectomy include the following: definitive diagnostic confirmation of an ovarian cyst, removal of symptomatic cysts, and exclusion of ovarian cancer. Additional indications include cyst size larger than 7.6 cm, cysts that do not resolve after 2-3 mo of close observation, bilateral lesions, and ultrasound imaging findings that deviate from a simple functional cyst. Note that both the patient's age at the time of detection and as well as cyst type can influence surgical indications as reviewed below.
Fetal
Ovarian cysts in the developing fetus are more common than previously thought owing to their detection by antenatal ultrasound imaging. They have been identified on routine obstetrical ultrasound in 30-70% of fetuses, with the frequency increasing as gestation advances. [15] They are usually unilateral and often resolve spontaneously. They originate as a consequence of ovarian stimulation from combined maternal and fetal gonadotropins. Surgical management, including cyst aspiration, is not usually indicated. [15, 16]
Neonatal
Ovarian cysts are thought to develop in neonates as a consequence of in utero hormonal stimulation . They are also more common than previously thought. Approximately 30% of neonates that underwent post mortem examination had ovarian cysts. [15] Most neonates are asymptomatic with the cysts usually identified by ultrasound for unrelated indications. Many are simple cysts while others are complex, rendering a benign diagnosis more difficult. They often regress spontaneously in the first 4-5 postnatal months. Cysts measuring greater than 5 cm are of concern in this age group as they often torsed and can auto amputate. Bryan et al recommend aspiration to prevent torsion from occurring. [15] Cystectomies are not usually indicated.
Prepubertal child
Ovarian cysts are usually seen in early childhood, age less than 6 yrs, and then again in the peripubertal period when the hypothalamic pulse generator is the most active. Gonadotropin stimulation of the ovary can cause some follicles to become cystic, with some cysts persisting. [17] Mature cystic teratomas (MCTs) represent 90% of all ovarian tumors in this age group. [3] Some cysts are autonomously functioning and may secrete hormones such as those seen in the McCune-Albright syndrome. [18] The presentation in the young child varies. Asymptomatic patients may present with a palpable abdominal mass or increasing abdominal girth, while symptomatic children may present with increasing abdominal pain. [17] McCune-Albright patients often present with signs indicative of precocious puberty. [18] Cystectomies are not usually indicated since most resolve spontaneously.
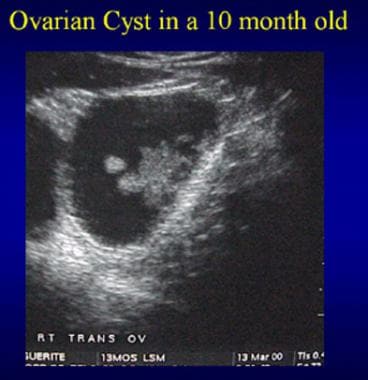
The images below depict an ovarian cyst in a 10-month-old girl.
Menarchal adolescents and adults
Benign ovarian cysts are quite common in menarchal adolescents and adults. Most regress within 2-3 months after detection, while others persist. Features associated with persistent ovarian cysts include cysts larger than 5 cm and complex morphologic findings on ultrasound.
The types of cysts that occur in reproductive age females differ from those encountered in early childhood. The most common benign ovarian cysts in this population are endometriomas and MCTs. Endometriomas are relatively common in the menarcheal teen and adult but are rarely seen in childhood. Patients with endometriomas often present with dysmenorrhea and dyspareunia. They may also present with pelvic pain, bloating, urinary frequency, menstrual irregularities, and/or constipation.
Young women with torsed ovarian cysts may present with nausea and vomiting along with acute abdominal pain and require surgical intervention. MCTs are the most common ovarian neoplasms in adolescents and account for nearly 70% of non-malignant ovarian neoplasms in females aged 30 yrs or younger. [19] Patients with MCTs are usually asymptomatic and present with unrelated complaints. Indeed, in this setting, the detection of MCTs is often an incidental finding. The most common complaint in symptomatic patients is abdominal pain. [20]
The most common benign ovarian cysts seen in pregnant women are MCTs and corpus luteum cysts. Acute complications have been reported to occur in less than 2% of these cases. [21, 22] Malignant ovarian cysts are infrequent in reproductive-age women, occurring in 3.6-6.8% of this population. [21]
Postmenopausal
Unilocular small (< 5 cm) ovarian cysts in postmenopausal women have a low risk for malignancy. The frequency for malignancy increases between 6-39% in this population when the cyst is large (>10 cm), has complex architecture (multilocular, thick septae, irregular cyst walls), or persists. [22] CA 125 measurements should be performed. Many postmenopausal women with large ovarian cysts are asymptomatic because menstrual irregularities and dysmenorrhea are no longer indices of pathology. When symptomatic, they can present with urinary frequency, constipation in addition to pelvic pain. [23]
The image below depicts a multilocular ovarian cyst.
Contraindications
Absolute contraindications for an ovarian cystectomy are controversial. Relative contraindications are related to the surgical approach rather than to the cystectomy proper. Indeed, any ovarian lesion suspicious for malignancy based on findings from the clinical history and/or physical examination, ultrasound studies, or elevated CA 125 levels is a contraindication for laparoscopic ovarian cystectomy, with laparotomy the procedure of choice.
Additional contraindications depend on the cyst type and the indication(s) for surgery as well as the approach to the patient. In the 1980s, the laparoscopic approach was contraindicated in obese patients as well as patients having previous abdominal surgeries, a bowel obstruction, or a coagulopathy. Currently, these conditions are considered relative contraindications. The use of the open laparoscopic technique as well as the use of optical access trocars has rendered the laparoscopic approach safe and feasible in many patients even if they have had previous surgeries.
Although no longer a contraindication, obesity does represent additional challenges. Not only is obesity a risk factor for anesthesia-related complications such as airway obstruction and cardiopulmonary dysfunction, but it also poses technical challenges for the surgeon. [24]
Anesthesia
General anesthesia is indicated for both the laparoscopic and robotic-assisted techniques, since both of these procedures require the surgeon to create a pneumoperitoneum. Although either spinal or epidural anesthesia can be used for a laparotomy cystectomy, in most cases general anesthesia is used.
Equipment
Video cameras/equipment
In performing laparoscopic surgery, having instruments and video equipment that enables the operator to perform the surgery to the best of his or her abilities is important. Most recently, the authors' university practice has been using the 1188 HD 3-chip camera (Stryker, Kalamazoo, MI 49002) that gives a 1280 x 1024 native output. In addition, the X8000 light source (Stryker, Kalamazoo, MI 49002) with a 300-watt xenon elliptical bulb has given us excellent results. The Vision Elect HDTV 26 monitor (Stryker, Kalamazoo, MI 49002) provides a clear crisp picture and has the advantages of providing a picture-in-picture and split-screen displays. Many fine digital documentation systems exist. The SDC Ultra digital camera (Stryker, Kalamazoo, MI 49002) works well in the stated system because it allows video capture and storage as well as DVD burning.
Surgical instruments
A number of laparoscopes are available and are made by various companies (Boston Scientific, Natick, MA; Genico, Inc. Winter Park, Fl; Precision Optics Corp., Kansas City, MO; Stryker, Kalamazoo, MI).
The most commonly used laparoscope by the authors' university practice is the 5 mm 0°, but in some cases the 10 mm 0° is also used. Other instruments used along with the laparoscope that have utility in performing ovarian cystectomies include the following: Kleppinger Bipolar Laparoscopic Forceps (Wolf, Vernon Hill, IL); curved scissors with a monopolar handle; various grasping forceps such as the wave or the Allis, atraumatic fenestrated, and Babcock; and a 5-mm blunt probe (Specialty Surgical Instrumentation, Antioch, TN). Additional instruments include ovarian biopsy forceps (VMS Medical, Inc., Indianapolis, IN), 5 mm x 32 cm StrykeProbe (Stryker, Kalamazoo, MI); ENDOPOUCH specimen retrieval bag and ENDOPATHÒ Uterine Manipulator (Ethicon, Cincinnati, OH); Kronner Uterine Manipulator (Artisan Medical, Medford, NJ).
Positioning
Patient positioning is contingent upon the surgical approach of the ovarian cystectomy. For laparoscopy and robotic-assisted techniques, the dorsal lithotomy position is the preferred position as it easily accommodates placement of a manipulator in the uterus for proper uterine positioning during the procedure. Either the dorsal lithotomy or the dorsal supine position can be used for laparotomy cystectomies. Uterine manipulators are not routinely used in this approach. It has been our experience that without the uterine manipulator, the uterus and ovaries can more easily be exteriorized during laparotomy cystectomies, thus allowing for working outside of the pelvis. In all cases, Foley catheter placement is important.
Technique
Expectant or surgical management of ovarian cysts is contingent upon the age of the patient, the cyst characteristics, the presence of symptoms and a suspicion of malignancy. A thorough preoperative evaluation of the patient with an ovarian cyst should in most cases distinguish benign from malignant lesions.
Laparoscopy
Laparoscopic cystectomy is the preferred approach to managing benign ovarian cysts in adolescents and adults. A retrospective study of 282 females aged 25 years or younger who underwent laparoscopic surgery concluded that the procedure is a safe first-line strategy for cysts in this age group. The intraoperative diagnosis of these cases was highly correlated with the final pathology. [25] As previously stated, any ovarian lesion suspicious for malignancy is a contraindication for laparoscopy.
The standard 3-port set up is most commonly used. Puncture sites include a para-umbilical and 2 side port incisions about 3 cm superior to the mons pubis lateral to the rectus muscles. In general, 5-mm ports can be used at all 3 sites. In some cases, a 10-12 port is needed to remove the cyst wall. The abdomen is insufflated with CO2 to 15 mm of mercury and the laparoscope is inserted. [26] Examination of all peritoneal surfaces, liver, and diaphragm is conducted and, when normal, the ovaries are identified and similarly examined.
Care should be rendered when incising the ovary so as not to spill the cyst contents. [27] Two common methods for the initial ovarian incision are electrocautery and harmonic scalpel. Once the initial incision is made, the cyst walls must be separated from the ovary using traction and blunt dissection. Aqua dissection with hydrostatic pressure often facilitates removal of the cysts without rupture. Once the intact cyst is freed from the ovary, it can be carefully opened with electrocautery and the contents aspirated to reduce the size of the mass.
The deflated, excised cyst wall can then be removed through one of the ports. In some cases, posterior vaginal colpotomy incision is used for removal of the intact cyst or cyst wall. [28] Another option is to place the cyst in an endoscopic retrieval bag and then decompress the cyst inside the bag. This procedure significantly decreases the possibility of spilling the cyst contents into the pelvic cavity. One study reported a 7.3-8.1% spillage rate for laparoscopic ovarian cystectomies. [29]
Laparoscopic excision of endometriomas causes a significant and progressive fall in serum antimüllerian hormone levels. [30] A prospective cohort study by Kwon et al, however, indicated that the rate of serum antimüllerian hormone decline following laparoscopic ovarian cystectomy is similar no matter which type of benign cyst is present. The study involved 100 women who underwent laparoscopic excision of endometriomas (68 patients) or other benign cysts (32 patients). The only factor significantly affecting the decline rate was whether or not the cysts were bilateral. [31]
Laparotomy
Although laparoscopic ovarian cystectomy has become the standard surgical approach for treating benign ovarian cysts, laparotomy continues to be an alternative approach. Indications for laparotomy include large, complex cysts that cannot be removed through the laparoscope, cysts for which the benign nature is under question, and for cysts in which spillage of contents would result in significant morbidity. [27]
Care must be taken to avoid cyst content spillage into the peritoneal cavity. Some surgeons recommend that the ovary be surrounded by a moist lap the time of cystectomy in case inadvertent rupture of the cysts occurs. An elliptical incision is carefully made through the ovarian cortex to the cyst wall. Once the cyst wall is reached, blunt and sharp dissection using either the knife handle or surgical scissors is used to separate the cyst wall from the surface of the ovary.
Some surgeons prefer to use fine-needle cauterization to develop the dissection plane and separate the cyst wall from the ovarian cortex using scissors. In most cases, the cyst can be separated from the ovary without rupture. The dead space of the cyst fossa is then closed in layers with 7-0 nonreactive absorbable sutures using vertical mattress or figure-of-8 stitching to approximate the ovarian wall. [26] In some cases with minimal bleeding, closing the cyst fossa is not necessary.
Robotic-assisted surgery
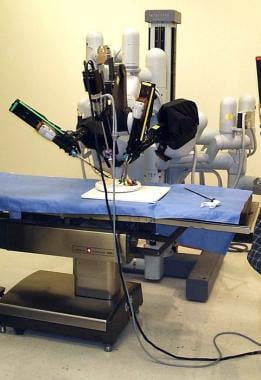
Robotic-assisted surgery is one of the latest innovations in the field of minimally invasive gynecologic surgery with FDA approval obtained in 2005 (da Vinci, Intuitive Surgical, Sunnydale, CA). A recent study by Nezhat et al evaluated a number of gynecologic procedures, including ovarian cystectomies, performed with robotic assistance and reported good outcomes and a number of advantages with this approach. [32]
The image below depicts the da Vinci Surgical System.
Some advantages of robotic surgery include 3-dimensional visualization, improved ergonomics, reduced fatigue, decreased tension tremor, 7º of motion, and decreased blood loss. [33] Added wrist motion for improved dexterity is an additional advantage. Overall, the advantages of better visualization can enhance proper dissection and preservation of ovarian tissue. However, robotic-assisted surgery is expensive, and some surgeons remark on the loss of tactility and time allotted for the assembly and disassembly. Thus, this approach may not be advantageous for all cases. Additional studies are needed to better define its advantages over laparoscopic ovarian cystectomies.
Ultrasound-guided aspiration
Ultrasound-guided cyst aspiration is often used in assisted reproductive technology. The most common indication in this setting is oocyte retrieval after controlled superovulation with gonadotropins. Often these cysts reaccumulate fluid because the follicular cells continue their secretory function.
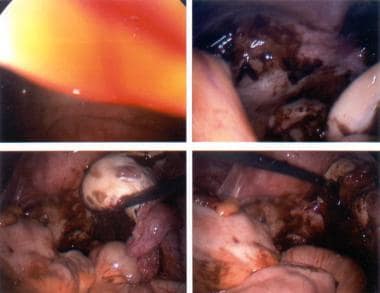
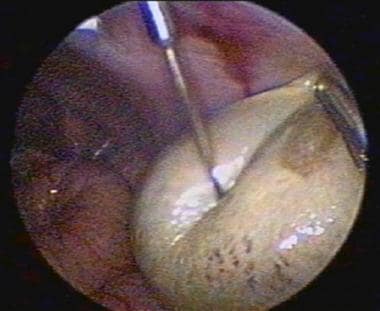
The image below depicts aspirating an ovarian cyst.
Ultrasound-guided cyst aspiration is also used to reduce persistent cysts following controlled super-ovulation. Since the cyst wall is not destroyed, fluid reaccumulation often occurs. The cure rate for cysts arising in these setting ranges from 30-80%. This approach is not usually used for treating persistent cysts of unknown origin, since no pathological specimen is obtained and the fluid reaccumulates. Other methods, such as sclerotherapy, have been reported to prevent reaccumulation of cyst fluid. In this approach, 5% tetracycline or methotrexate is injected into the cyst fossa after aspiration of the contents. Aspirated cyst fluid can be sent for cytological examination, but it is often inconclusive.
Pearls
Before attempting an ovarian cystectomy, properly evaluating the patient to ensure that the proper approach is used is important. This is particularly important when the cyst is suspected to be malignant. In such cases, consulting with oncology prior to surgery and making them available for back up is imperative. In cases involving neonates, the expertise of a pediatric surgeon may be required. Although laparoscopy is the criterion standard approach for benign ovarian cystectomies, laparotomy may be the preferred method when a substantial risk of spillage of cystic contents exists, as with some cystic teratomas or potential malignancies.
Complications
Complications of ovarian cystectomies are generally related to bleeding and inadvertent cyst rupture intraoperatively. Bleeding can usually be controlled with electrocautery. Ovarian preservation is essential for patients desiring future fertility, so not destroying a large portion of the ovary while achieving hemostasis is important. The addition of hemostatic agents such as Surgicel fibrillar, Evisel, Surgiflo (Ethicon, Inc., Somerville, NJ), or Floseal and Gelfoam (Baxter International, Inc., Deerfield, IL) in some cases may be appropriate.
The frequency of intraoperative cyst rupture at laparoscopic cystectomy ranges from 6-27% and occurs more frequently than at laparotomy. [28, 29] Inadvertent spillage of the contents of an endometrioma may result in subsequent spread of the condition to other parts of the pelvis. Spillage of the contents of a cystic teratoma may result in peritoneal irritation, while the rupture of a malignant cystic structure is more serious. It may result in tumor dissemination and adversely affect the patients' survival. [28] When spillage occurs, the pelvis should be copiously irrigated.
-
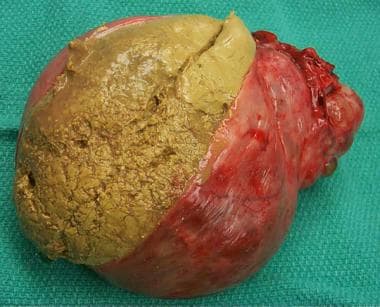
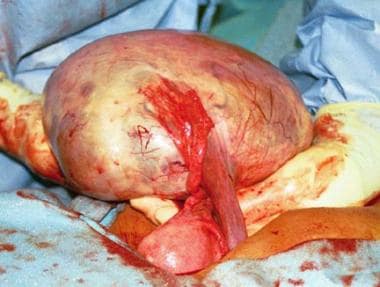
A 24-cm diameter multilocular right ovarian cyst is seen with adjacent fallopian tube and uterus. The infundibulopelvic ligament carrying the ovarian artery and vein has been divided.
-
Endometriosis. Chocolate cyst of the ovary.
-
Mature cystic teratoma of the ovary exhibiting multiple tissue types.
-
Mature cystic teratoma of the ovary with hair, sebaceous material, and thyroid tissue.
-
A laparoscopic robotic surgery machine. Patient-side cart of the da Vinci Surgical System.
-
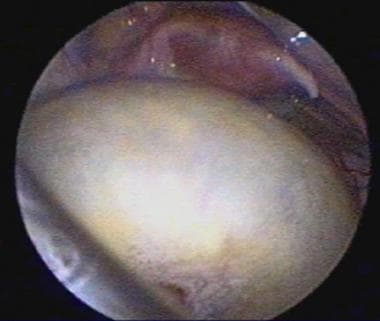
Ovarian cyst in a 10-month-old girl. The uterus and tubes are visible in the pelvis.
-
Ultrasound of an ovarian cyst in a 10-month-old girl.
-
Aspirating an ovarian cyst.